|
A sinister conspiracy?
During 2008, the European Commission conducted a “Pharmaceutical Sector Inquiry” that was published I November 2008, but whose findings have only become widely known during early 2009.
In its Executive Summary, the Commission explains why it launched the enquiry.
“In January 2008 the European Commission launched a sector inquiry into EU pharmaceuticals markets under the EC competition rules (Articles 81 and 82 of the EC Treaty) because information relating to innovative and generic medicines suggested that competition may be restricted or distorted. This was indicated by a decline in innovation measured by the number of novel medicines reaching the market, and instances of delayed market entry of generic medicines, as compared to what might be expected.”
To me it seemed as though the Commission had decided what results it wanted to find and then set out to uncover the evidence to support its conclusions.
So I looked at the evidence that they had assembled and conclude that they were partly right and partly wrong. Let’s go through the points in the order that they raised them.
A decline in innovation measured by the number of novel medicines reaching the market.
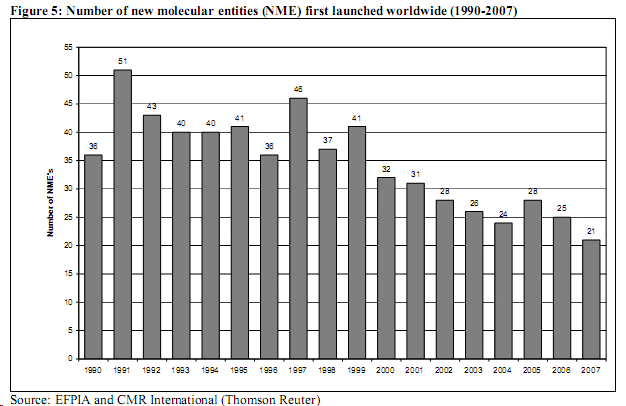
When I looked at this, the first thought that occurred to me was to wonder why the Commission might think that any Big Pharma company in its right mind would voluntarily not bring a potential blockbuster to market. What sense would it make to cut off your own source of future income? The report included a table that clearly shows a decline in introduction of NCEs since 1990 – hardly news to anyone that works in the pharmaceutical industry. However, after that as far as I can see, the report does not seem to say much about why this lack of innovation might be sinister perhaps because there is no logical reason why and R&D company would want to hold back from launching its new product.
Instead, it concentrates mainly on why generics are slow to reach the market, focussing on the one hand on deals between Big Pharma and generic companies and on the other hand on fights between Big Pharma and generic companies.
Big Pharma – generic conspiracies
It might come as a great surprise to readers to learn that there have in fact been cases where the originator has paid generic companies to stay off the market! I touched on this sort of behaviour in the US in a previous article “How to make more money by not launching” where I wrote “It might seem perverse and illogical, but many US generic firms have discovered a really good way of making money from generics. They refrain from launching the product.”
The European Commission has concluded that the same happens in Europe. It stated in regard to settlements between originator and generic company “…. 23 agreements (51%) included a payment from the originator company to the generic company, 29 settlements (64%) included a licence, nine cases (20%) included a supply and/or distribution agreement..” and included the following chart.
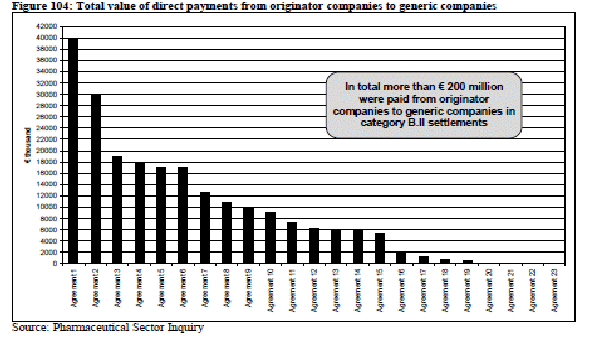
The report defined B.II settlements as “Agreements that limited the generic company's ability to market its own product and included a value transfer to the generic company”. The percentages add up to more than 100% because some settlements included more than one element. It is in this area particularly where the Commission appears to see a conspiracy.
Big Pharma patent strategies
It also looked at the more familiar area of originators using patent strategies to try to deflect or delay generic entry. There was particular interest in the way that some R&D companies try to persuade regulators to take the patent situation into account even though in the report’s words “Under EU law, linking the granting of marketing authorisation for a product to the patent status of an originator company's reference product is unlawful”. The report added, “the sector inquiry revealed that a significant number of interventions is taking place every year, in particular in relation to high-turnover products”
In this context the following quote is particularly interesting:
“An adviser to an originator company described such approaches to marketing authorisation bodies as follows:
"Certain Health Authorities will provide [the originator company with] information about pending applications [of generic companies] for marketing authorisation: some formally […], some informally. […] Establishing a good rapport is essential – you are on their side! […] separate functions of persuading/challenging if this helps.”
However, the investigators also weaken their case for a conspiracy to delay generic entry by referring to a far more serious cause of delays, which is the bureaucracy of many authorities when it comes to granting prices, and deciding on reimbursement. At its recent conference, the EGA identified these two factors of intervention with regulators and reimbursement delays as particularly problematic.
So if you work for a generic company and think that they are out to get you – you could be right!
For those who are interested, the full report is available from the EU Commission at:
http://ec.europa.eu/competition/sectors/pharmaceuticals/inquiry/index.html
If you have any questions or comments,
Please feel free to contact me
peter@interpharm-consultancy.co.uk
www.interpharm-consultancy.co.uk
|